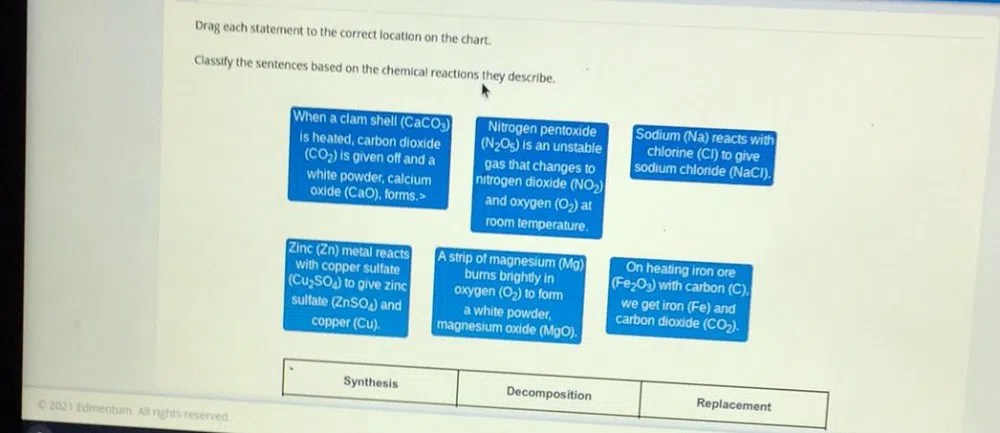
Classify the sentences based on the chemical reactions they describe, a fundamental concept in chemistry, provides a systematic approach to understanding the diverse range of chemical transformations. This comprehensive guide delves into the intricacies of chemical reactions, exploring their types, characteristics, and practical applications.
By classifying chemical reactions, scientists gain valuable insights into the behavior of atoms, molecules, and ions, enabling them to predict reaction outcomes, design new materials, and advance scientific knowledge.
Sentence Classification Based on Chemical Reactions: Classify The Sentences Based On The Chemical Reactions They Describe
In chemistry, reactions are classified based on various criteria. Understanding the types of chemical reactions helps chemists predict the products, reaction mechanisms, and potential applications.
The main types of chemical reactions include:
- Combination reactions
- Decomposition reactions
- Single-replacement reactions
- Double-replacement reactions
- Combustion reactions
- Redox reactions
Each type of reaction has its own unique characteristics, reactants, and products.
Combination Reactions, Classify the sentences based on the chemical reactions they describe
Combination reactions occur when two or more substances combine to form a single product. The general form of a combination reaction is:
A + B → AB
For example, when hydrogen and oxygen gases combine, they form water:
2H 2+ O 2→ 2H 2O
Decomposition Reactions
Decomposition reactions are the opposite of combination reactions. In a decomposition reaction, a single compound breaks down into two or more simpler substances. The general form of a decomposition reaction is:
AB → A + B
For example, when water is electrolyzed, it decomposes into hydrogen and oxygen gases:
2H 2O → 2H 2+ O 2
Single-Replacement Reactions
Single-replacement reactions occur when one element replaces another element in a compound. The general form of a single-replacement reaction is:
A + BC → AC + B
For example, when iron reacts with copper sulfate, iron replaces copper in the compound, forming iron sulfate and copper:
Fe + CuSO 4→ FeSO 4+ Cu
Essential FAQs
What are the different types of chemical reactions?
Chemical reactions are broadly classified into several types, including synthesis, decomposition, single displacement, double displacement, and combustion reactions.
How are chemical reactions classified?
Chemical reactions are classified based on various criteria, such as the number and type of reactants and products, the nature of the chemical change, and the energy changes involved.
What are the practical applications of classifying chemical reactions?
Classifying chemical reactions has numerous practical applications, including predicting reaction outcomes, designing new materials, developing pharmaceuticals, and understanding environmental processes.